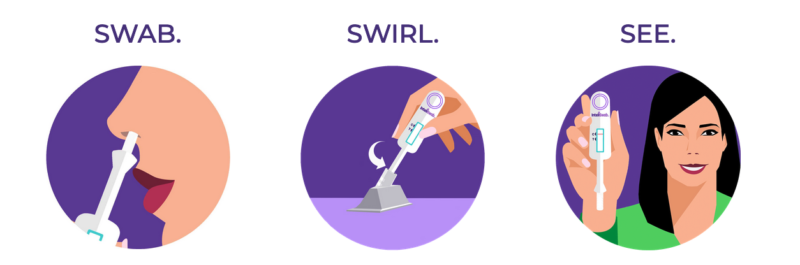
InteliSwab™ COVID-19 Rapid Test is an antigen test. Antigen tests detect proteins (small parts) from the SARS-CoV-2 virus. Antigen tests are designed to detect virus levels that reflect active infection. There are no confusing steps, no batteries and no mailing to labs. You just swab your nostrils with the gentle swab, swirl it in the tube, and see results in minutes. It’s just that simple. That’s the power of InteliSwab™. It provides test results in minutes without the need for sending your test sample out to a laboratory.
InteliSwab™ COVID-19 Rapid Test (Over-the-Counter)
The InteliSwab™ COVID-19 Rapid Test is a type of test called an antigen test. COVID-19 antigen tests are designed to detect proteins from the virus that causes COVID-19 respiratory specimens, for example in anterior nasal swab specimens. The InteliSwab™ is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms tested twice over three days with at least 36 hours between tests.
InteliSwab™ COVID-19 Rapid Test Pro (Point-of-Care)
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
The InteliSwab™ COVID-19 Rapid Test Pro is authorized for use with anterior nasal samples from individuals 18 years or older when the sample is self-collected or in individuals 15 years or older when the sample is collected by an adult or healthcare provider. The test is authorized for individuals who are suspected of COVID-19 by their healthcare provider within 7 days of symptom onset or from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests.
The InteliSwab™ COVID-19 Rapid Test Pro is for use under the Food and Drug Administration’s Emergency Use Authorization (EUA) only. The InteliSwab™Pro is intended for use by medical professionals or trained operators who are proficient in performing tests and trained clinical laboratory personnel or individuals trained in POC settings.
How accurate is InteliSwab™?
InteliSwab™ is a lateral flow in vitro diagnostic antigen test to detect COVID-19. Antigen tests are designed to detect active infection in individuals. A clinical study was conducted during February and April of 2021 to determine the performance of the InteliSwab™ test.
A total of 146 individuals with signs and symptoms of COVID-19 within the first 7 days of symptom onset were enrolled across 5 different locations in the US. Subjects 18 years or older independently collected the lower nasal sample and completed the home use test.
InteliSwab™ results were compared to highly sensitive molecular FDA Authorized SARS-CoV-2 assays to determine test performance. InteliSwab™ correctly identified 84% of the positive samples with both low levels and high levels of virus.
Additionally, InteliSwab™ correctly identified 98% of negative samples.
Does this test detect the new SARS-CoV-2 variants?
“OraSure Technologies, Inc. …announced … that its InteliSwab® COVID-19 rapid tests detect the Omicron BA.2, BA.2.12.1, BA.3 and BA.5 subvariants with similar levels of detection to the original SARS-CoV-2 strain and other previous variants of concern, including Delta, Alpha, Beta, Gamma and Omicron BA.1. The Omicron sublineage studies were conducted using live SARS-CoV-2 virus at an independent, third-party laboratory and showed InteliSwab® detected the Omicron variants at similar viral load levels, or limit of detection, as previous variants that were tested.”
Citation: OraSure Technologies – InteliSwab® COVID-19 Rapid Test Validated to Detect the Omicron BA.2, BA.2.12.1, BA.3 and BA.5 Subvariants. (n.d.). OraSure Technologies. Retrieved October 11, 2022, from https://orasure.gcs-web.com/news-releases/news-release-details/inteliswabr-covid-19-rapid-test-validated-detect-omicron-ba2