Product Description
Flowflex COVID-19 Antigen Home Test is a rapid test for the detection of SARS-CoV-2 antigens in anterior nasal specimens directly from individuals within 7 days of symptom onset or without symptoms or other epidemiological reasons to suspect COVID-19 infection. For self-testing use. For use under an Emergency Use Authorization (EUA) only.
FDA UPDATE:
FDA has granted 510(k) clearance for Flowflex and becomes the first FDA 510(k) for an OTC rapid antigen Covid test! The 510(k) version of Flowflex will be produced domestically.
OMICRON UPDATE: Does Flowflex Detect Omicron?
Clinical Performance:
The performance of Flowflex COVID-19 Antigen Home Test was established in an all-comers clinical study conducted between March 2021 and May 2021 with 172 nasal swabs self-collected or pair-collected by another study participant from 108 individual symptomatic patients (within 7 days of onset) suspected of COVID-19 and 64 asymptomatic patients. The Flowflex COVID-19 Antigen Home Test results were compared to an FDA EUA RT-PCR COVID-19 assay to determine test performance in the table below:
Flowflex COVID-19 Antigen Home Test |
RT-PCR method | ||
| Positive | Negative | Total | |
| Positive | 39 | 0 | 39 |
| Negative | 3 | 130 | 133 |
| Total | 42 | 130 | 172 |
| Positive Percent Agreement (PPA) | 93% (95% CI: 81% -99%) | ||
| Negative Percent Agreement (NPA) | 100% (95% CI: 97% – 100%) | ||
Analytical Sensitivity: Limit of Detection (LoD): LoD was determined as the lowest virus concentration that was detected ≥ 95% of the time. Based on this testing, the LoD in nasal matrix was confirmed to be 2.5 x 103 TCID50/mL
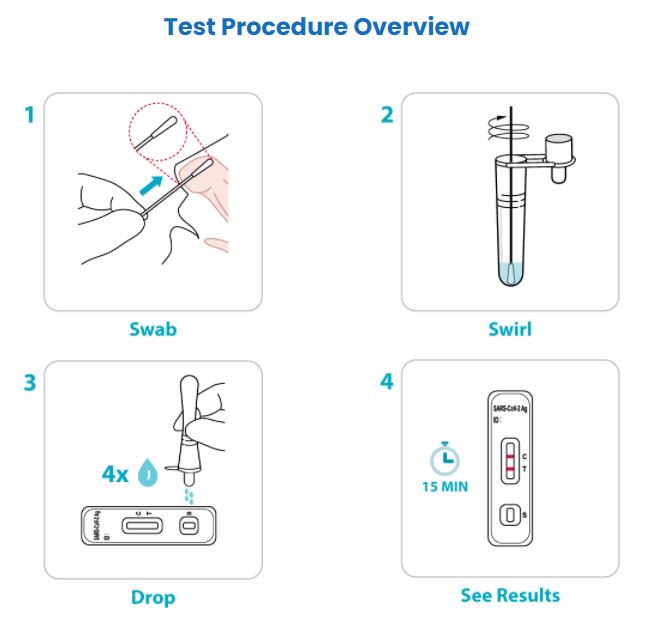
IMPORTANT: This test procedure overview does not replace the package insert. Before you begin the test, it is important to carefully read and follow the detailed instructions in the package insert. Failure to follow the instructions may result in inaccurate test results.
CPT Code for COVID-19: 87811QW
FEATURES & BENEFITS
- Cassette Format
- Detects the nucleocapsid protein antigen from SARS-CoV-2
- Nasal Specimen sample
- Results in 15 minutes
- FDA Emergency Use Authorization (EUA)
- Positive Percent Agreement (PPA): 93% (95% Cl: 81%-99%)
- Negative Percent Agreement (NPA): 100% (95% Cl: 97%-100%)
- Analytical Sensitivity: Limit of Detection (LoD): 2.5 x 103 TCID50/mL
PRODUCT DETAILS
You may place order by giving us a call
| Catalog Number | Description | Units of Measurements |
| IDL031118B5 | Flowflex™ COVID-19 Antigen Home Test | 1 Test/Kit |
| IDL031125M5 | Flowflex™ COVID-19 Antigen Home Test | 2 Tests/Kit |
| IDL031125P5 | Flowflex™ COVID-19 Antigen Home Test | 25 Tests/Kit |
DOCUMENTATION AND VIDEOS
Flowflex COVID-19 Antigen Home Test videos, brochure, package inserts, authorized FDA letter and additional literature provided below:
Video Instructions – Adult
Video Instructions – Child
Jant Pharmacal is an Authorized Distributor for the Flowflex COVID-19 Antigen Home Test.
This product has not been FDA cleared or approved but has been authorized by FDA under an EUA.
This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of IVDs for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.
For more information on EUAs please visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19