Product Description
Status™ COVID-19/Flu test is a lateral flow immunoassay intended for the in vitro rapid,
simultaneous qualitative detection and differentiation of nucleocapsid antigen from SARS-CoV2, influenza A and influenza B directly from anterior nasal and nasopharyngeal swab specimens obtained from individuals, who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider, within the first five days of onset of symptoms. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests.
This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Kit Includes
25 Test Devices
25 Extraction Reagents in Capsules
25 Sterile Swabs
1 Positive and 1 Negative Control Swabs
1 Package insert
1 Quick Reference Instruction
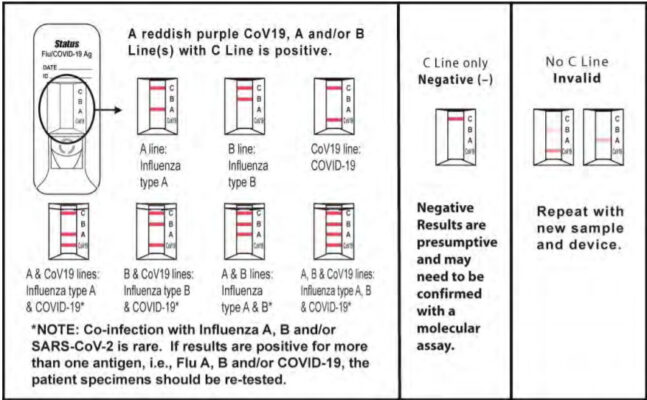
Results Interpretation
Positive: Determination of a positive result is made at fifteen (15) minutes. A reddish purple Control line (C position) and a reddish purple Test line (Flu A, Flu B or CoV19 position) indicate that Influenza A, B and/or SARS-CoV-2 antigen has been detected. Lines at the A and C positions indicate the presence of Influenza type A viral antigen, lines at the B and C positions indicate the presence of Influenza type B viral antigen, and lines at the CoV19 and C positions indicate the presence of SARS-CoV-2 viral antigen in the specimen. A positive result does not rule out co-infections with other pathogens or identify any specific influenza A virus subtype.
Negative: A reddish purple Control line (C position) only, with no Test line at the A, B, CoV19 positions, indicates that Influenza A, B antigen or SARS-CoV-2 antigen has not been detected. A negative result does not exclude influenza viral or SARS-CoV-2 viral infection. Determination of negative results should not be made before 15 minutes.
Invalid: A reddish purple line should always appear at the Control line position (C
position). If a line does not form at the Control line position in 15 minutes, the test result
is invalid and the test should be repeated with a new Status™ COVID-19/Flu test device.
IMPORTANT: Please refer to package insert (instructions for use) for more comprehensive product information.
CPT Codes for COVID-19 and Flu A/B:
COVID-19: 87811QW
FLU A: 87804QW
FLU B: 87804QW-59
FEATURES & BENEFITS
- COVID-19
– Anterior nasal swab specimen − Sensitivity 93.8 %, Specificity 100%
– Nasopharyngeal − Sensitivity 93.1 %, Specificity 100%
- Flu A – Sensitivity 91.4%, Specificity 95.7%
- Flu B – Sensitivity 87.6%, Specificity 95.9%
- FDA Emergency Use Authorization (EUA)
- Visually read in 15 minutes
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
PRODUCT DETAILS
You may place order by giving us a call
| Catalog Number | Description | Units of Measurements |
| ID33225 | Status COVID-19 and FLU A/B | 25 Tests/Kit |
DOCUMENTATION AND VIDEOS
The Status COVID-19/Flu A&B Antigen Rapid Test Kit brochure, product insert, authorized Fact Sheet for Healthcare Providers, authorized Fact Sheet for Recipients and additional studies are provided below: