QuickVue At-Home OTC COVID-19 Test lets you get rapid results, in the privacy of your own home. Available over-the-counter, everything you need is in the package and taking the test is simple.
This home test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older. The test to be used in individuals within 6-days of symptom onset or individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
Effectively Detects Omicron & Other Variants
The Quickvue At-Home OTC COVID-19 test can accurately detect the Omicron variant (among others) of the Sars-CoV-2, the potentially deadly virus that can lead to COVID-19.
How Does the QuickVue At-Home OTC COVID-19 Test Work?
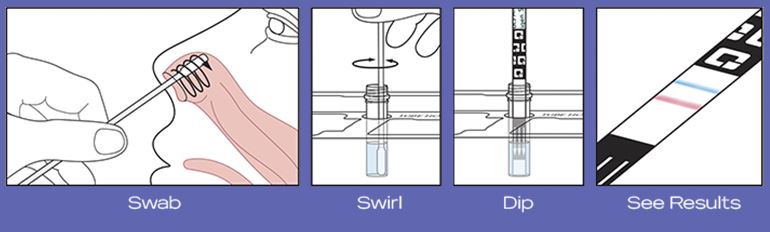
The test uses a gentle self-collected anterior nasal (nares) swab sample to determine a positive or negative COVID-19 result. The swab is swirled in a tube of reagent solution, then removed, before a test strip is inserted. After ten minutes, you can take the strip out of the tube and see your results.
Here’s a quick overview of how it works:
IMPORTANT: Before you begin the test, it’s important to first read and closely follow the detailed instructions, included in the package..
CPT Code for COVID-19: 87811QW
FEATURES & BENEFITS
- Test Strip Format
- Detects the nucleocapsid protein antigen from SARS-CoV-2
- Nasal Specimen sample
- Results in 10 minutes
- FDA Emergency Use Authorization (EUA)
PRODUCT DETAILS
You may place order by giving us a call
| Catalog Number | Description | Units of Measurements |
| QV20402 | QuickVue At-Home OTC COVID-19 Test | 2 Tests/Kit |
DOCUMENTATION AND VIDEOS
The QuickVue At-Home OTC COVID-19 Test is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals within 6-days of symptom onset or individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
This test is authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 14 years or older with symptoms of COVID-19 within the first six days of symptom onset. This test is also authorized for non-prescription home use with adult collected anterior nasal swab samples from individuals aged 2 years or older with symptoms of COVID-19 within the first six days of symptom onset.
This test is also authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older, or adult collected anterior nasal swab samples from individuals aged 2 years or older, with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
This product has not been FDA cleared or approved; but has been authorized by FDA under an Emergency Use Authorization (EUA) This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
For more information on EUAs please visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19