Product Description
The Accutest Saliva Cotinine Test is a lateral flow chromatographic immunoassay for the qualitative detection of cotinine in oral fluids at a cut-off concentrations of 30 ng/mL.
The Accutest Saliva Cotinine Test is a rapid, oral fluid screening test that can be performed without the use of an instrument. The test utilizes antibodies to selectively detect elevated levels of specific drugs in human oral fluid.
Cotinine is the first-stage metabolite of nicotine, a toxic alkaloid that stimulates the autonomic ganglia and central nervous system in humans. Nicotine is a drug to which virtually every member of a tobacco-smoking society is exposed whether through direct contact or second-hand inhalation. Aside from tobacco, nicotine is also commercially available as the active ingredient in smoking replacement therapies such as nicotine gum, transdermal patches and nasal sprays.
Regardless of whether nicotine in a donor was derived from tobacco use or through a nicotine-replacement therapy, if the metabolite cotinine is present in sufficient concentration, the test result will be positive.
Although nicotine is excreted in saliva, the relatively short half-life of the drug makes it an unreliable marker for tobacco use.
Cotinine, however, demonstrates a substantially longer half-life than nicotine, bears a high correlation with plasma cotinine levels and has been found to be the best marker for smoking status compared with saliva nicotine measurements, breath carbon monoxide testing and plasma thiocyanate testing.
The window of detection for cotinine in saliva at a cutoff level of 30 ng/mL is expected to be up to 1-4 days after nicotine use.
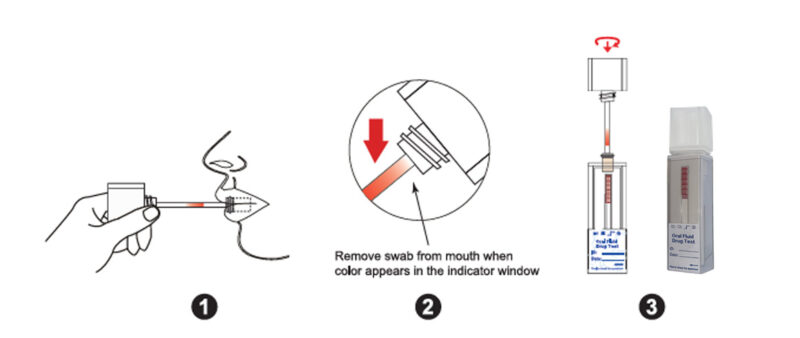
SIMPLE COLLECTION AND TEST PROCEDURE:
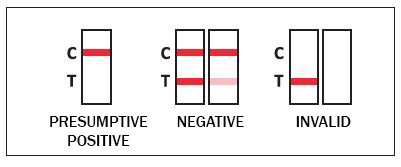
RESULTS INTERPRETATION:
PRELIMINARY POSITIVE: A colored line in the control line region (C) but no line in the test line region (T) for a specific drug indicates a positive result. This indicates that the drug concentration in the oral fluid specimen exceeds the designated cut-off for that specific drug.
NEGATIVE: A colored line in the control line region (C) and a colored line in the test line region (T) for a specific drug indicates a negative result. This indicates that the drug concentration in the oral fluid specimen is below the designated cut-off level for that specific drug.
INVALID: Control line (C) fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure.
*This test provides only a preliminary result. A more specific alternative chemical method should be used to obtain a confirmed presumptive positive result if the donor doesn’t admit use or anytime required by testing procedures. Gas Chromatography/ Mass Spectrometry (GC-MS), Liquid Chromatography/ Mass Spectrometry (LC-MS) and their tandem mass-spectrometer versions are the preferred confirmatory methods. Careful consideration and judgment should be applied to any drug screen test result, particularly when evaluating preliminary positive results.
FEATURES & BENEFITS
- Over-the-counter (OTC)
- CLIA Categorized: Waived*
- Rapid, On-Site Determination of Nicotine Use
- Results in 10 minutes
*This product can be used by CLIA Waived facilities
PRODUCT DETAILS
You may place order by giving us a call
| Catalog Number | Description | Units of Measurements |
| DS443 | Accutest® Saliva Cotinine Test | 25 tests/box |
DOCUMENTATION AND VIDEOS
Brochure, package inserts and additional literature provided below: